The Solid-Liquid Extraction Method
What is solid-liquid extraction?
Solid-liquid extraction, the cornerstone of countless scientific breakthroughs, traces its roots to the very inception of scientific research. Having evolved from the simple distillation methods of ancient civilizations to the sophisticated techniques, such as Soxhlet extraction, that are made possible with our modern instruments, the importance of extraction is indisputable. At BUCHI, we are dedicated to keeping the spirit of this evolution alive, driving the future of food and feed, environmental and chemical, and broader laboratory sectors through our state-of-the-art extraction instruments.
Today, extraction techniques, such as solid-liquid extraction, Soxhlet extraction, and pressurized solvent extraction, are instrumental across various fields of study, from sample preparation for analysis of complex mixtures to environmental testing, food quality control, and regulatory compliance, to name a few. Our comprehensive portfolio of instruments reflects this diversity of applications and provides solutions with precision, speed, safety, and reliability.
With extensive experience in extraction technology, BUCHI shapes the trajectory of scientific innovation by offering advanced instrumentation, services, and support by partnering with laboratories worldwide to meet the most challenging needs.
Exploring standard extraction methods
There are various methods for extraction. One of the most traditional and widely used methods is Soxhlet extraction, developed in the 19th century by Franz von Soxhlet, which involves the continuous extraction of a sample by distillation. Besides the traditional classical extraction methods, newer extraction methods are available that are faster and drastically reduce solvent consumption. One way of increasing efficiency is to increase the extraction temperature. Two methods that utilize this influence are hot extraction (Randall extraction) and economic continuous extraction (Twisselmann extraction). More recently, increasing pressure has further improved efficiency, as with the Pressurized Solvent extraction (PSE) method.
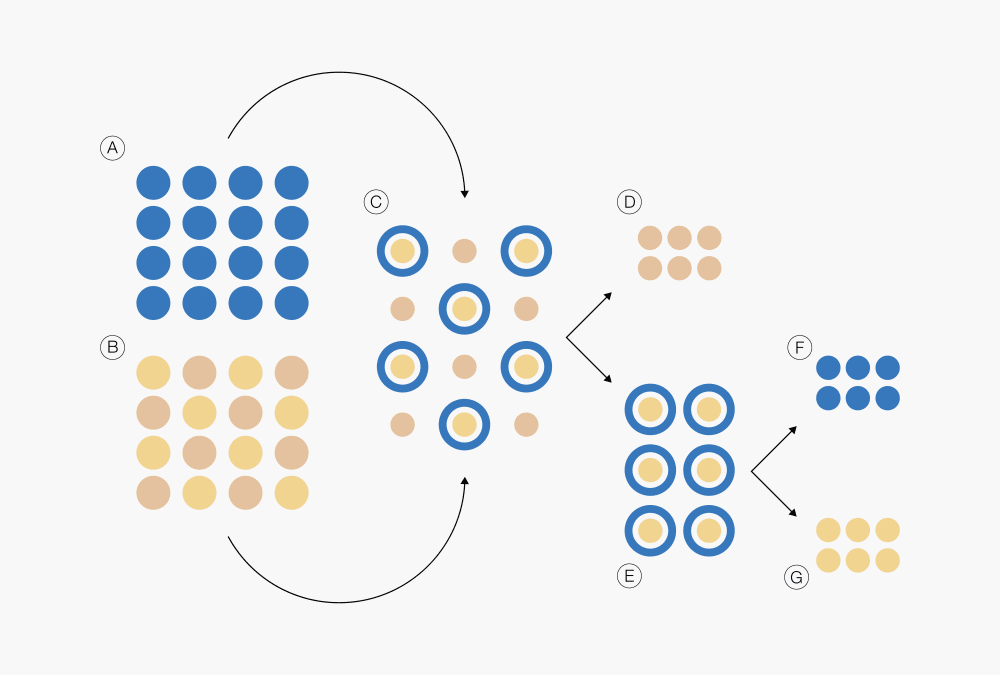
Figure 1: Extraction process
Ⓐ Extraction solvent
Ⓑ Extraction sample
Ⓒ Extraction mixture
Ⓓ Extraction residue
Ⓔ Extraction solution
Ⓕ Extraction solvent
Ⓖ Extract
How Soxhlet extraction works
This solid-liquid extraction method allows continuous sample extraction by ongoing solvent distillation, enhancing efficiency. The homogenized sample is a mixture of solvent-soluble and insoluble components. For extraction, the sample is placed into a thimble. The solvent is separated from the sample, heated, and evaporated.
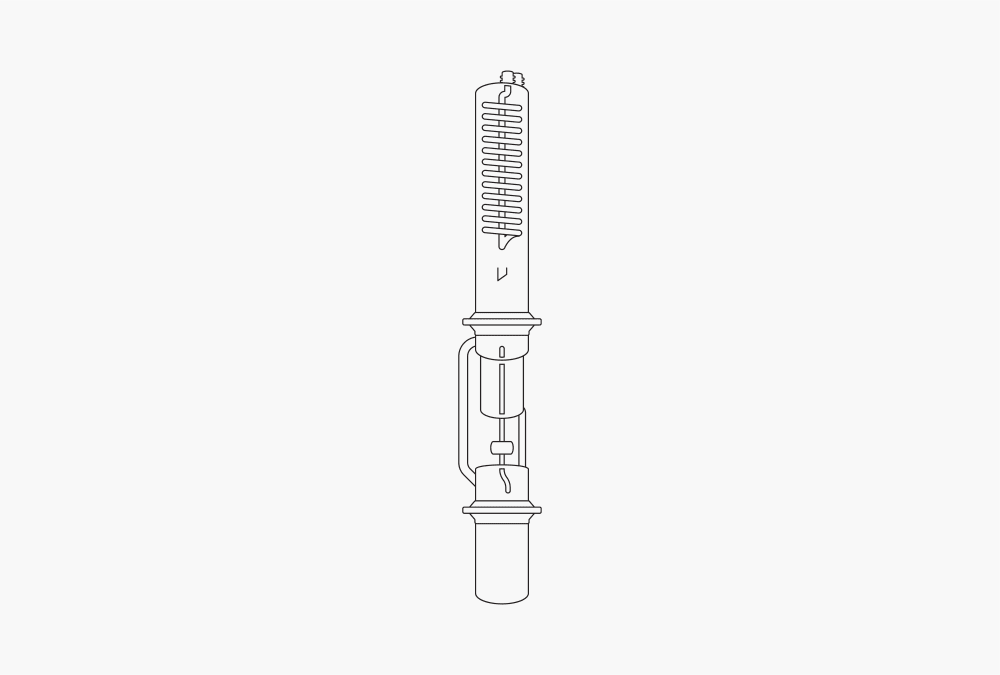
Figure 2: Soxhlet extraction
The condensed solvent is mixed with the sample. Once the extraction thimble is filled completely with solvent, the extract is siphoned, and the proportions of soluble components are separated from the sample. Other soluble and insoluble components remain in the sample. If this procedure is repeated several times, the sample is continuously extracted with a freshly distilled solvent. The proportion of extracted soluble components increases while the insoluble component remains in the residue. The repetition of the siphoning is called a cycle. The temperature of the Soxhlet extraction is limited to the solvent’s boiling point.
How hot extraction works
In this method, also known as Randall extraction, the samples are placed directly in the beaker and immersed in the boiled solvent. Therefore, the sample and solvent are not separated from each other. This procedure introduces a higher temperature into the sample than Soxhlet extraction, increasing the extraction efficiency. The soluble component is released from the sample and collected in the beaker through subsequent solvent evaporation.
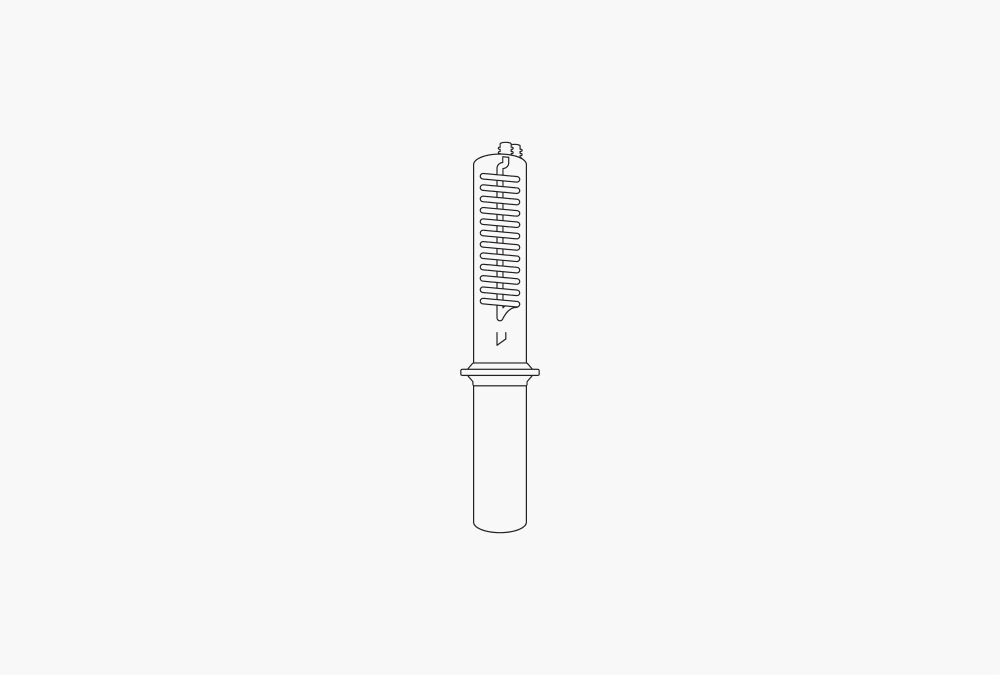
Figure 3: Hot extraction
How Twisselmann extraction works
In economic continuous extraction (ECE), the sample is separated from the solvent, as it is in Soxhlet extraction. But different from the Soxhlet extraction, the solvent is not collected in the extraction chamber. ECE involves the sample being kept in the hot solvent vapor while being efficiently rinsed with freshly distilled solvent. The combination of hot vapor and freshly distilled solvent increases the extraction efficiency.
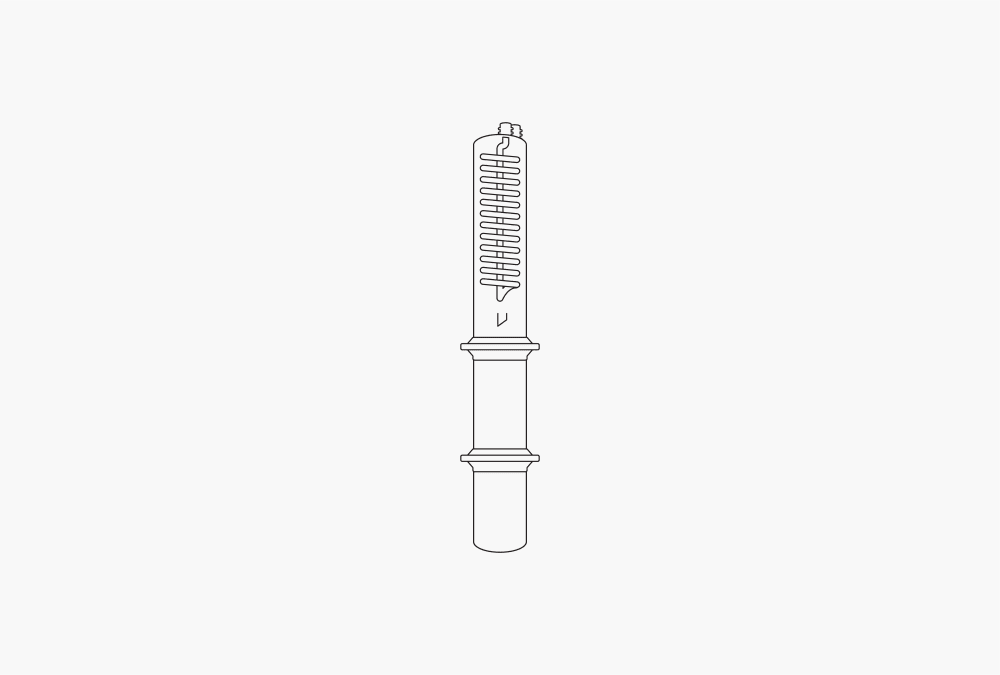
Figure 4: Economic continuous extraction
How pressurized solvent extraction works
In solid-liquid extraction, this method involves samples being extracted at elevated pressures. This increase in pressure allows for raising temperatures above the solvent's boiling point, increasing extraction efficiency. A higher temperature increases the solubility of components and the solvent’s viscosity. The solvent can absorb a larger proportion of components with each extraction cycle. The samples are placed in stainless steel cells, and the solvent mixture is transferred by a high-pressure liquid chromatography (HPLC) pump at high pressure (< 150 bar) and temperature (< 200 °C) settings. Depending on the application, the sample is submerged under elevated pressure in a hot solvent for the appropriate amount of time. When the extraction is finished, the extracts are collected in vials and can be concentrated for further analysis.
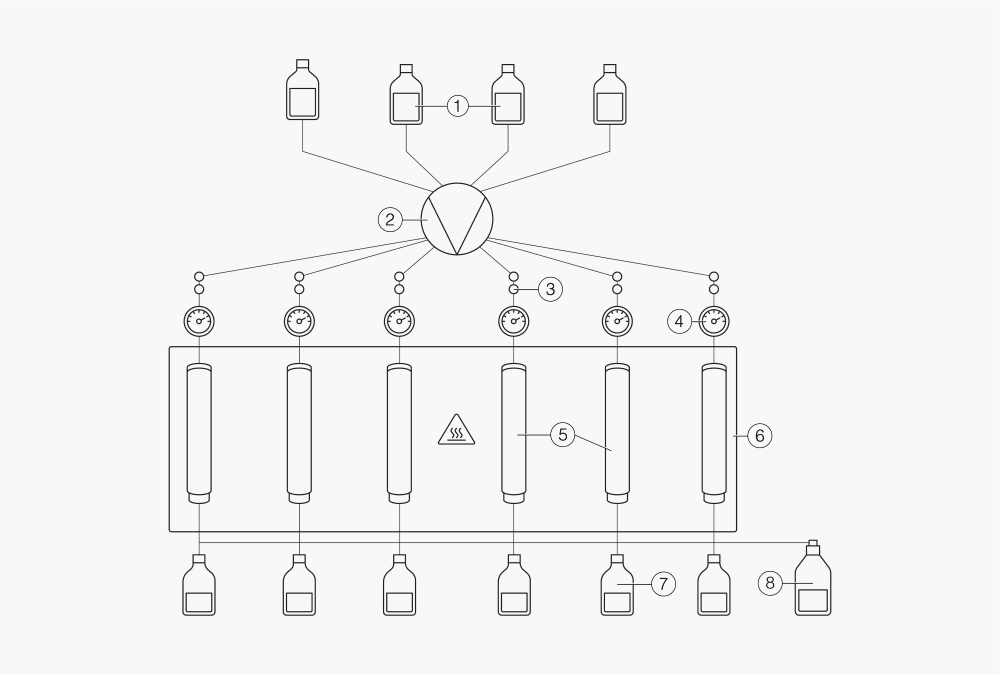
Figure 5: Pressurized solvent extraction
① Solvent reservoirs
② HPLC pump
③ Position valves
④ Pressure gauges
⑤ Extraction cells
⑥ Heating block
⑦ Collection bottles
⑧ Waste bottles
Considering the influencing factors
An initial important factor to consider is that the extraction solvent must always be inert with regard to the extraction substance. Many other parameters also influence the recoveries and the speed of the extraction. The most important points to consider when optimizing the extraction process are listed in the table below:
Table 1: Influences on the extraction process
| Factors that influence the recovery rate of the extraction | Factors that influence the speed of extraction |
|---|---|
Solubility of the components to be extracted in the selected extraction solvent (must be of similar polarity)
| The particle size of the extraction substance |
The thoroughness of the mixing of extraction substance with the extraction solvent
| The degree to which extraction substance and extraction solvent are mixed |
Size and number of extraction solvent portions (number of siphons in the case of Soxhlet or drop rate of solvent in other methods) | Temperature (rule of thumb is that speed of reaction doubles for every 10 °C temperature increase) |
| Nature of sample (enclosed fat, moisture, size, surface, homogeneity) |
One of the best strategies to ensure you are using optimal extraction parameters is to apply one of the standard methods mentioned. These processes contain validated and recognized extraction methods and parameters, which help to increase the reliability of your extraction workflow.
How to reach endpoint determination
During the extraction process, the concentration of soluble components in the extraction substance decreases steadily until it reaches a point where continuing the extraction is of no further value. This point is called the practical conclusion. The amount of extraction solvent (number of siphons) required for the extraction to reach the point of practical conclusion mostly depends on the solubility of the substance being extracted. Although in many cases, determining the practical conclusion is a matter of experience, there are approaches that can help assess if the point has been reached. One of the most common ways to determine the end of the extraction is to verify the extraction method using reference material with known content.
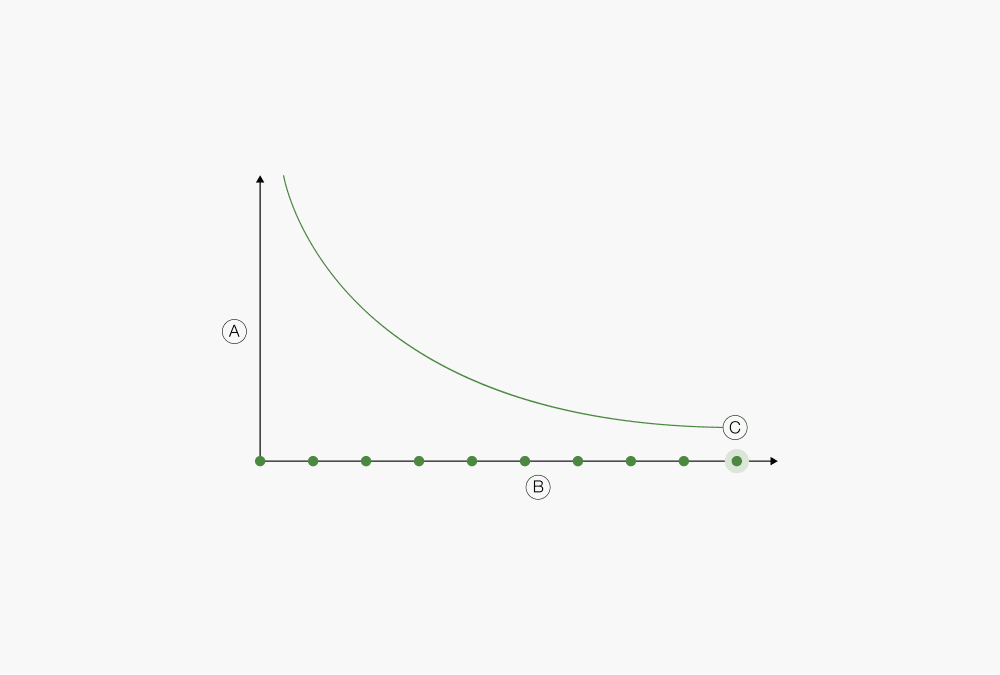
Figure 6: Determination of extraction endpoint
Ⓐ Concentration of soluble components in the sample
Ⓑ Number of solvent portions
Ⓒ Practical conclusion